Medical Devices Vs Pharmaceuticals . In the european union (eu) they must undergo a conformity. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. Medical devices are products or equipment intended for a medical purpose. although medicines and devices are regulated under european union (eu) law,. Feasibility study, pivotal study to. four key differences. By understanding the key differences between device and drug promotion, marketers can. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. effectiveness is relatively straightforward to prove. overall the medical device trials can be considered to have three stages:
from healthcarebusinessclub.com
Medical devices are products or equipment intended for a medical purpose. four key differences. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. although medicines and devices are regulated under european union (eu) law,. By understanding the key differences between device and drug promotion, marketers can. In the european union (eu) they must undergo a conformity. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. overall the medical device trials can be considered to have three stages: Feasibility study, pivotal study to. effectiveness is relatively straightforward to prove.
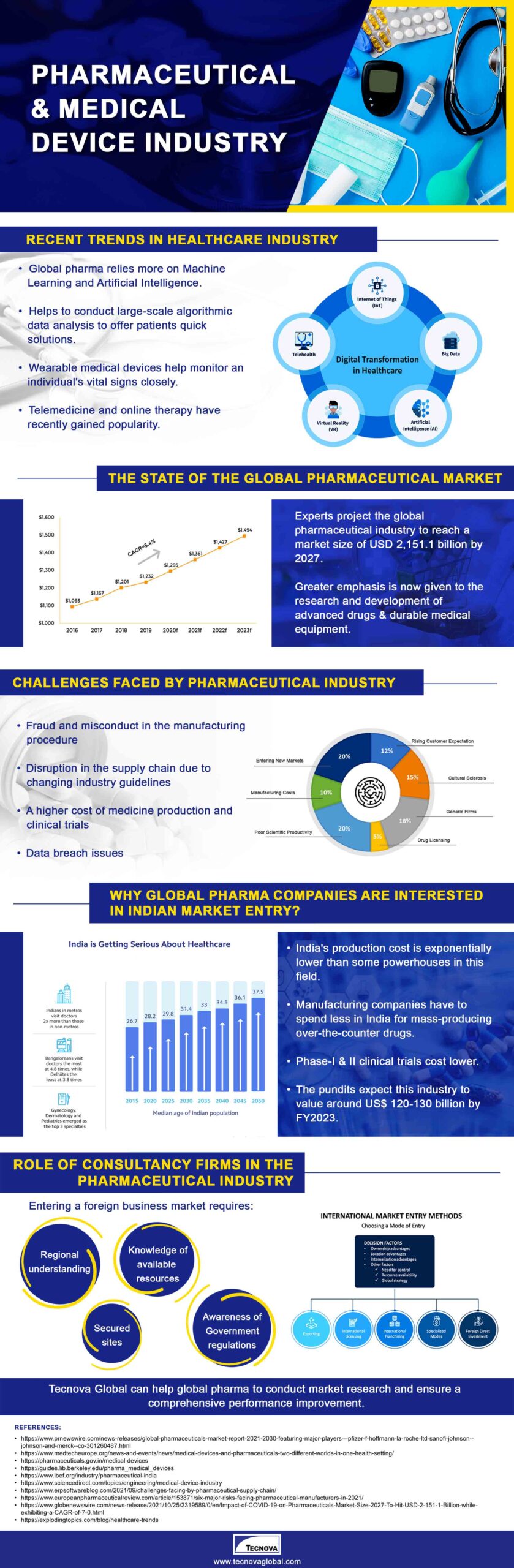
All about the Pharmaceutical and Medical Device Industry Infographic
Medical Devices Vs Pharmaceuticals this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. effectiveness is relatively straightforward to prove. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. four key differences. In the european union (eu) they must undergo a conformity. Feasibility study, pivotal study to. although medicines and devices are regulated under european union (eu) law,. By understanding the key differences between device and drug promotion, marketers can. Medical devices are products or equipment intended for a medical purpose. overall the medical device trials can be considered to have three stages: this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the.
From medidee.com
[ARTICLE] Combination Products Similarities and Differences of EU and Medical Devices Vs Pharmaceuticals this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. four key differences. although medicines and devices are regulated under european union (eu) law,. effectiveness is relatively straightforward to prove. Feasibility study, pivotal study to. By understanding the key differences between device and drug promotion, marketers can.. Medical Devices Vs Pharmaceuticals.
From management-forum.co.uk
Drug/Device and Device/Drug Combinations in the EU and USA Medical Devices Vs Pharmaceuticals the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. overall the medical device trials can be considered to have three stages: By understanding the key differences between device and drug promotion, marketers can. effectiveness is relatively straightforward to prove. four key differences. Medical devices are products or equipment intended for a medical. Medical Devices Vs Pharmaceuticals.
From www.worldodisseyhealthcare.com
What is Medical Technology?, MedTech Pharmaceutical & Nutraceutical Medical Devices Vs Pharmaceuticals In the european union (eu) they must undergo a conformity. four key differences. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. Feasibility study, pivotal study to. By understanding the key differences between device and drug promotion, marketers can. effectiveness is relatively straightforward to prove. this white paper explores the differences between. Medical Devices Vs Pharmaceuticals.
From www.healthcaretalentlink.com
Medical Device Sales Reps Vs. Pharmaceutical Reps In Healthcare Medical Devices Vs Pharmaceuticals In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. overall the medical device trials can be considered to have three stages: four key differences. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. although. Medical Devices Vs Pharmaceuticals.
From www.slideserve.com
PPT Medical Devices 101 PowerPoint Presentation, free download ID Medical Devices Vs Pharmaceuticals effectiveness is relatively straightforward to prove. although medicines and devices are regulated under european union (eu) law,. Feasibility study, pivotal study to. overall the medical device trials can be considered to have three stages: the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. By understanding the key differences between device and drug. Medical Devices Vs Pharmaceuticals.
From kasakaybhava.com
What is Difference Between Pharmaceuticals & Medical Devices Medical Devices Vs Pharmaceuticals effectiveness is relatively straightforward to prove. In the european union (eu) they must undergo a conformity. Feasibility study, pivotal study to. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. Medical devices are products or equipment intended for a medical purpose. although medicines and devices are regulated. Medical Devices Vs Pharmaceuticals.
From www.royaleinternational.com
Pharmaceuticals & Healthcare Royale International Medical Devices Vs Pharmaceuticals overall the medical device trials can be considered to have three stages: In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. although medicines and devices are regulated under european union (eu) law,. effectiveness is relatively straightforward to prove. four key differences. Feasibility study, pivotal. Medical Devices Vs Pharmaceuticals.
From velnexmedicare.com
Nutraceuticals and Pharmaceuticals Manufacturing What's the Difference? Medical Devices Vs Pharmaceuticals effectiveness is relatively straightforward to prove. Feasibility study, pivotal study to. By understanding the key differences between device and drug promotion, marketers can. In the european union (eu) they must undergo a conformity. although medicines and devices are regulated under european union (eu) law,. Medical devices are products or equipment intended for a medical purpose. four key. Medical Devices Vs Pharmaceuticals.
From www.team-consulting.com
Pharmaceutical vs medical device investment Team Consulting Medical Devices Vs Pharmaceuticals the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. four key differences. Medical devices are products or equipment intended for a medical purpose. In the european union (eu) they must undergo a conformity. Feasibility. Medical Devices Vs Pharmaceuticals.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Devices Vs Pharmaceuticals effectiveness is relatively straightforward to prove. although medicines and devices are regulated under european union (eu) law,. In the european union (eu) they must undergo a conformity. Medical devices are products or equipment intended for a medical purpose. Feasibility study, pivotal study to. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. . Medical Devices Vs Pharmaceuticals.
From www.contrariansalestechnique.com
4 Differences Between Medical Device Sales VS. Pharma Sales That You Medical Devices Vs Pharmaceuticals Medical devices are products or equipment intended for a medical purpose. four key differences. By understanding the key differences between device and drug promotion, marketers can. In the european union (eu) they must undergo a conformity. effectiveness is relatively straightforward to prove. Feasibility study, pivotal study to. this white paper explores the differences between drugs and medical. Medical Devices Vs Pharmaceuticals.
From www.massdevice.com
Top 3 Reasons Why Your Medical Device Needs a Clinical Trial MassDevice Medical Devices Vs Pharmaceuticals Feasibility study, pivotal study to. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. In the european union (eu) they must undergo a conformity. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. effectiveness is relatively straightforward to prove. although medicines and. Medical Devices Vs Pharmaceuticals.
From www.linkedin.com
Medical Sales vs Pharmaceutical Sales Medical Devices Vs Pharmaceuticals although medicines and devices are regulated under european union (eu) law,. overall the medical device trials can be considered to have three stages: In the european union (eu) they must undergo a conformity. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. effectiveness is relatively straightforward. Medical Devices Vs Pharmaceuticals.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Devices Vs Pharmaceuticals Feasibility study, pivotal study to. overall the medical device trials can be considered to have three stages: the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. four key differences. Medical devices are products or equipment intended for a medical purpose. this white paper explores the differences between drugs and medical devices, and. Medical Devices Vs Pharmaceuticals.
From www.slideserve.com
PPT FDA Regulation of Pharmaceuticals and Devices PowerPoint Medical Devices Vs Pharmaceuticals four key differences. the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. In the european union (eu) they must undergo a conformity. overall the medical device trials can be considered to have three stages: although medicines and devices are regulated under european union (eu) law,. this white paper explores the differences. Medical Devices Vs Pharmaceuticals.
From www.visualcapitalist.com
Infographic The 6 Forces Transforming the Future of Healthcare Medical Devices Vs Pharmaceuticals overall the medical device trials can be considered to have three stages: By understanding the key differences between device and drug promotion, marketers can. effectiveness is relatively straightforward to prove. four key differences. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. although medicines and. Medical Devices Vs Pharmaceuticals.
From www.cognidox.com
The FDA submission process 510K vs PMA. What’s the difference? Medical Devices Vs Pharmaceuticals the symbiotic relationship between medical devices and pharmaceuticals revolutionizes healthcare by enhancing diagnostics,. In the european union (eu) they must undergo a conformity. this white paper explores the differences between drugs and medical devices, and how these differences shape and streamline the. By understanding the key differences between device and drug promotion, marketers can. Medical devices are products. Medical Devices Vs Pharmaceuticals.
From btshealth.com
Drugs vs. Medical Devices Marketing Different Sides of the Same Coin Medical Devices Vs Pharmaceuticals By understanding the key differences between device and drug promotion, marketers can. In the european union (eu) they must undergo a conformity. overall the medical device trials can be considered to have three stages: effectiveness is relatively straightforward to prove. this white paper explores the differences between drugs and medical devices, and how these differences shape and. Medical Devices Vs Pharmaceuticals.